
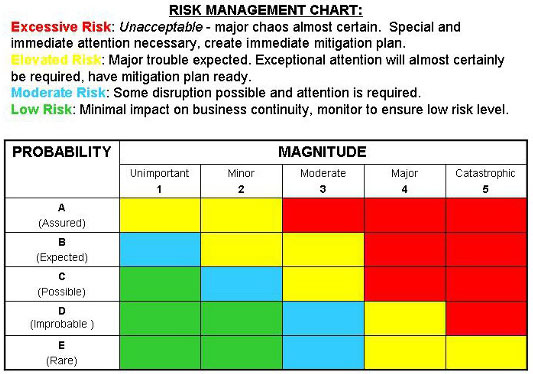
You may also need to conduct your own usability testing, which will allow you to see potential harms in action with real users. For starters, you’ll need to research what other medical device manufacturers have noted as harms resulting from each hazardous situation.

The harm in that case could be damage to the car - the property.įiguring out the harms at play for your product can be laborious. Harms describe damage to people, property, or the environment because of a hazardous situation. Your documented hazards and hazardous situations should be listed in a hazard analysis table - a spreadsheet that tracks all pertinent risk management details - before you move on in the process.Īfter documenting hazards and hazardous situations in your hazard analysis table, it’s time to discern the potential harms associated with those hazardous situations. A related hazardous situation could be a car driving over that pothole. And a hazardous situation is the scenario that exposes users or the environment to one or more hazards.įor clarity, consider a non-medical device example. You may also find this article about how to develop a medical device risk management plan helpful.Ī hazard refers to a potential source of harm. You can find much of the information you need to document hazards and hazardous situations in ISO 14971:2019 Medical Devices - Application of risk management to medical devices. Hazards and Hazardous Situations: A Recapĭetermining hazards and hazardous situations is an important initial step during your risk management journey. Let’s explore the steps that should happen after you’ve determined the hazards associated with your forthcoming product.

It starts at the beginning of your development project and never ceases - unless your device is taken off of the market.Įven though the risk management process is continuous, you can break it down into steps to make it more manageable for you and your team. Not to mention the risk management process itself is never-ending. It’s a lot of pressure to account for and mitigate risks so your medical device is both safe for users and meets the FDA’s exacting requirements. The risk management process for medical devices can be overwhelming.


 0 kommentar(er)
0 kommentar(er)
